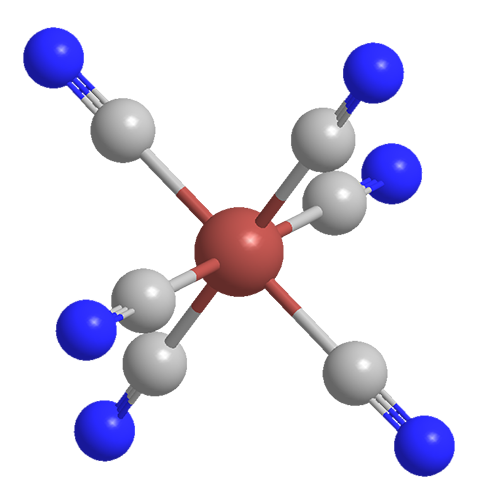
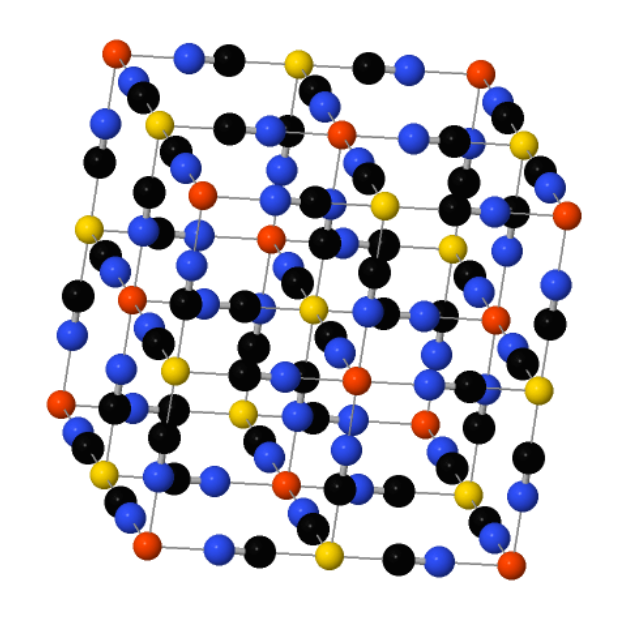
'Prussian blue' is a crystal so blue you can't accurately show it on most computer screens, since they can only display a limited region of color space. Its structure is really cool. It's a cubical lattice made of iron atoms, each surrounded by 8 cyanides - carbon and nitrogen. But let Sean Silver explain it:
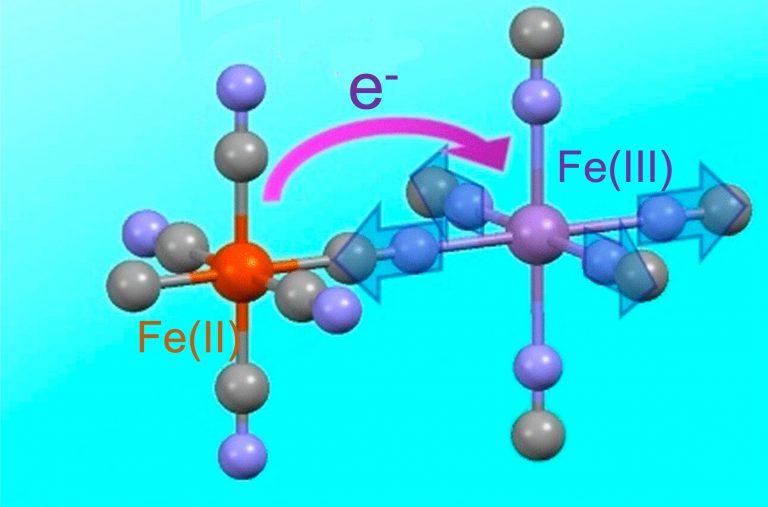
"The modern way of manufacturing the pigment involves synthesizing it directly from some form of hexacyanoferrate; hexacyanoferrate is one iron atom bound with six cyanide molecules radiating equidistantly from it, like the tiny metal doodads scooped up in the children’s game called “jacks.” These are snapped into a theoretically endless lattice, the point of each hexacynoferrate compound lining up with a point of another, which are locked into place by iron ions with a different charge. So: if we were to describe what we saw along any single axis, we would see iron(II), cyanide, iron(III), cyanide, iron(II), and so on.
Neither of the precursors to Prussian Blue is blue. And, though the very word “cyanide” comes from the Greek word meaning “blue,” this proves to be a backformation from Prussian Blue; in roughly 1750, cyanide was isolated as its own (deadly) compound by cracking it out of the pigment, and named “blue” despite the fact that it is nearly colorless. Hexacyanoferrate therefore has the very word “blue” in its name, though, by itself, it is hardly blue at all.
Understanding the color of Prussian Blue requires a short detour. I have become interested, lately, in situations where a whole is different from the sum of its parts – and that is the case with Prussian Blue, where blueness is an emergent effect of combination."
https://sites.rutgers.edu/motley-emblem/prussian-blue/
(1/n)